Wrought Iron - Hand Forged By Master Blacksmith
It has been our experience, that when most customers are shopping for wrought iron products, the correct pre-sales questions are seldom asked - which can result in them making a poor choice and a loss of their investment. Here at ArtFactory.com, we take pride in educating our customers about the products and processes used in creating their handcrafted wrought iron furnishings, gates, or hardware. We offer our customers full transparency, with the correct information and sources available, to allow the facts about how we create each custom iron product to be evaluated without the sales hype.
How it's made and what it is made from is paramount in almost all products. It becomes even more important when purchasing a new residential or commercial metal gate, chandelier, hardware item, or furnishing. You should always question the value of the item you are purchasing, not just make your selection based on a "pretty" design. How it's made, the raw materials it's made from, can make all the difference in a quality investment furnishing. Keep in mind that this choice of manufacturing determines your value in regards to how long your metal product will last. Also, how much maintenance will be necessary for the product's longevity. 
All high quality, hand forged wrought iron products are never welded, hollow steel, or cold bent.
The best iron products are handmade by a master blacksmith using a hot forge, anvil and hammer, and with hand applied patina finishes, built to last forever with no maintenance required - there is never a need to powder coat or paint real wrought iron.
Be aware that most other companies will try to keep you focused on the design, color, and cheaper price - not the merits of how their lower quality products are made. Why? Because if they disclosed the facts regarding their fast-process painted or powder coated steel or aluminum products, they wouldn't get your business. Most of these other manufacturers try to compare their "design" with our high quality, solid, hand-forged by a master-blacksmith products, in hopes of dazzling you with the similarity of their fast process, powered coated or painted designs, on the cheap. Keep in mind, if you're not comparing apples to the "same" apples, you are not getting a bargain, you're simply getting fooled by unscrupulous operators. However, if you are comparing products equally - based on materials and manufacturing processes - you will find that our factory direct prices are more than competitive. If you take the time to read and understand most manufacturers' "limited" warranties you will then have all the real facts.
Our Purpose for Educating You with the Genuine Facts on How It's Made
We believe our customers are smart, and if educated with solid, proven, indisputable information necessary to know the difference between the fake and faux that, armed with these facts, our customers will choose us to be their manufacturer. We also believe our customer, after receiving their handcrafted custom designed wrought iron product, will refer us to their friends and family and build upon a developing relationship of mutual respect, thus making this age old quote true again:
"BY HAMMER AND HAND DO ALL QUALITY THINGS STAND"
Iron - Coal Fired, Hammered by Master Blacksmith "The Old Fashioned Way"
At ArtFactory.com we take pride in our traditional, superior quality workmanship, and hand craft our custom designer products from the finest iron. Products are created by master blacksmith, who has been classically trained, and utilize old world techniques such as coal firing and anvil hammering. Solid wrought iron is hand forged to create the finest handcrafted, fine art, custom wrought iron gates, hardware, iron furniture and furnishings, and many other architectural elements hand forged in America.
Table of Contents
- - H. J. Nick - Artist Designer
- - Investment Quality Is Important
- - Guarantee & Satisfaction Warranty
- - How it's Made
- - Fine Furnishings Begin with Natural Materials
- - How It's Made - Wood Products
- - Master Hand Carving Information
- * How Its Made "Iron" - Master Blacksmithing
- - Facts About Leather Furniture
- - Stone, Marble & Granite
- - Art Factory Fine Art Finish Procedures
- - Finishes To World Class Antiquity Standards
- - American Vintage Restoration
- - More Door Details / Installation Info
- - Original Art or Limited Edition
- - Become an Interior Designer
- - A True Story About Mary
- - Dealer Design Policies & Rules
- - Web Site Terms And Conditions
- - Policies and Terms - A Must Read
Through fine arts and master craftsmanship, all our lives and personal histories can be elevated to a higher level and better standard. Many of the finest designs found throughout history have been copied by modern, cheap, fast production manufacturers. Navigating through the fake and faux designs can be a daunting task in today's marketplace. The best materials and master blacksmith techniques cannot be replaced with modern, fast process methods, so be sure to select a manufacturer like us, who utilizes only the finest materials and true artisan craftsmanship in our production process.
Contributors of the following information, derived from years of collective knowledge and hundreds of hours of volunteer work, consist of architectural university students and many experts from around the world who are interested in advancing the lost art of quality, hand made by master craftsmen furnishings in America and our non profit foundation for the advancement of the American fine arts and craftsman movement for future generations to enjoy. We employ the largest workforce of fine art blacksmiths in America. 
Metallurgy - The Historical Facts
Wrought Iron: Iron alloy phases v eFerrite (α-iron, δ-iron; soft) Austenite (γ-iron; harder) Spheroidite Pearlite (88% ferrite, 12% cementite) Bainite Martensite Ledeburite (ferrite-cementite eutectic, 4.3% carbon) Cementite (iron carbide, Fe3C; hardest)
Steel Classes: Carbon steel (≤2.1% carbon; low alloy) Stainless steel (+chromium) Maraging steel (+nickel) Alloy steel (hard) Tool steel (harder)
Wrought iron is commercially pure iron.
In contrast to steel, it has a very low carbon content. It is a fibrous material due to the slag inclusions (a normal constituent). This is also what gives it a "grain" resembling wood, which is visible when it is etched or bent to the point of failure. Wrought iron is tough, malleable, ductile and easily welded. Examples of items that used to be produced from wrought iron include: rivets, chains, railway couplings, water and steam pipes, raw material for manufacturing of steel, nuts, bolts, horseshoes, handrails, straps for timber roof trusses, boiler tubes, and fine art ornamental ironwork, furniture, doors, gates and hardware.
Wrought iron is no longer produced on a commercial scale. 
Many products described as wrought iron, such as guard rails, are made of mild steel. They retain that description because they were formerly made of wrought iron or have the appearance of wrought iron. True wrought iron is occasionally required for the authentic conservation of historic structures and fine art blacksmithing.
Wrought iron is so named because it is worked from a bloom of porous iron mixed with slag and other impurities.
The word "wrought" is an archaic past tense form of the verb to work. As irregular past-tense forms in English have historically been phased out over long periods of time, wrought became worked. Wrought iron literally means worked iron. Another theory is that "wrought" is the past tense of "wring".
Wrought iron is a general term for the commodity.
It is also used more specifically for finished iron goods, as manufactured by a master blacksmith or other smith. It was used in this narrower sense in British Customs records, such manufactured iron being subject to a higher rate of duty than what might be called "unwrought" iron.
Everything that Appears to Be Genuine Wrought Iron - May Not Be True Wrought Iron
More Hand Forged Iron Info
Defective quality iron is redshort if it contains sulfur in excess quantity. It has sufficient tenacity when cold, but cracks when bent or finished at a red heat. It is therefore useless for welding or forging.
Iron is coldshort (or "coldshear" or "colshire" or "bloodshot") if it contains phosphorus in excess quantity. It is very brittle when it is cold. It cracks if bent. It may, however, be worked at high temperature. Historically, coldshort iron was considered good enough for nails. Nevertheless, phosphorus is not necessarily detrimental to iron.
Ancient Indian smiths did not add lime to their furnaces; the absence of CaO in the slag, and the deliberate use of wood with a high phosphorus content during the smelting, induces a higher P content (> 0.1%, average 0.25%) than in modern iron. There is more phosphorus as solid solution throughout the metal than in the slags (one analysis gives 0.10% in the slags for 18% in the iron itself, for a total P content of 0.28% in the metal). This high P content and particular repartition are essential factors in the formation of a passive protective film of "misawite" (d-FeOOH), an amorphous iron oxyhydroxide that forms a barrier by adhering next to the interface between metal and rust.
 This Delhi iron pillar, made of 98% wrought iron, has withstood corrosion for the last 1600 years,
This Delhi iron pillar, made of 98% wrought iron, has withstood corrosion for the last 1600 years,
proof that pure wrought iron can stand the test of time.
From this technology recently rediscovered by metallurgists at IIT Kanpur through the study of the Iron Pillar of Delhi, rust-proof iron is in the last stages of being commercialized. This 1600 years-old rust-proof pillar is also of a remarkable strength, having withstood the impact of a cannon ball in the 18th century. Copper has a similar effect as phosphate regarding the formation of a passive protection film. Furthermore, the presence of phosphorus (without carbon) produces a ductile iron suitable for wire drawing, as for piano wire.
Iron Ore Processing
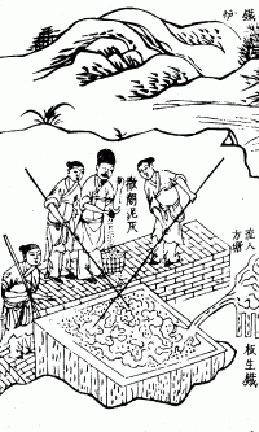
The puddling process of smelting iron ore - To make wrought iron from pig iron, the illustration to the left displays men working a blast furnace. Tiangong Kaiwu encyclopedia published in 1637, written by Song Yingxing (1587"1666).
Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout western history. The other form of iron, cast iron, was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could only be used for a limited number of purposes. Throughout much of the Middle Ages iron was produced by the direct reduction of ore in manually operated bloomeries, although waterpower had begun to be employed by 1104.
The raw material produced by all indirect processes is pig iron. It has a high carbon content and, as a consequence, it is brittle and could not be used to make hardware. The osmond process was the first of the indirect processes, developed by 1203, but bloomery production continued in many places. The process depended on the development of the blast furnace, of which medieval examples have been discovered at Lapphyttan, Sweden and in Germany.
The bloomery and osmond processes were gradually replaced in the 15th century by finery processes, of which there were two versions: the German and Walloon. They were in turn replaced in the late 18th century by puddling, with certain variants such as the Swedish Lancashire Process. These too are now obsolete, and wrought iron is no longer manufactured commercially, except one brand, "Pure Iron", which is made for artist blacksmiths and restorations of older ironworks.
Wrought Iron Varieties and Taxonomy
In the 17th, 18th and 19th centuries, wrought iron went by a wide variety of terms according to its form, origin, or quality.
- Bar iron - iron in bars, which are the usual product of the finery forge, but not necessarily made by that process. These might be square or flat, and flat bars might be narrow or broad.
- Rod iron - cut from flat bar iron in a slitting mill to provide the raw material for nails.
- Hoop iron - suitable for the hoops of barrels, apparently made by passing rod iron through flat rolls.
- Plate iron - sheets of iron suitable for use as boiler plate.
- Black-plate - sheets of iron, perhaps thinner than plate iron, from the black rolling stage of tinplate production.
- Voyage iron - narrow flat bar iron, made or cut into bars of a particular weight, a commodity for sale in Africa for the Atlantic slave trade. The number of bars per ton gradually increased from 70 per ton in the 1660s to 75"80 per ton in 1685 and "near 92 to the ton" in 1731.
- Oregrounds iron - a particularly pure grade of bar iron made ultimately from iron ore from the Dannemora mine in Sweden. Its most important use was as the raw material for the cementation process of steel-making.

- Danks iron - originally iron imported to Great Britain from Danzig (now Gdansk), but in the 18th century more probably the kind of iron (from eastern Sweden) that once came from Danzig.
- Forest iron - iron from the Forest of Dean, where haematite ore enabled tough iron to be produced.
- Lukes iron - iron imported from Liage, whose Dutch name is "Luik."
- Ames iron or amys iron - another variety of iron imported to England from northern Europe. Its origin has been suggested to be Amiens, but it seems to have been imported from Flanders in the 15th century and Holland later, suggesting an origin in the Rhine valley. Its origins remain controversial
- Botolf iron or Boutall iron - from Butow (Pommerania) or Beuthen (Silesia).
- Sable iron (or Old Sable) - iron bearing the mark (a sable) of the Demidov family of Russian iron-masters, one of the better brands of Russian iron.
- Tough iron - also spelt "tuf".
- Blend iron- made using a mixture of different types of pig iron.
- Best iron - in the 19th century, iron that had gone through several stages of piling and rolling, might reach the stage of being best iron.
- Marked Bar iron - iron made by members of the Marked Bar Association and marked with the maker's brand mark as a sign of its quality.
Ironworking Processes Throughout History
Bloomery Process
 Wrought iron was originally produced by a variety of smelting processes, all described today as bloomeries. Different forms of bloomery were used at different places and times. The bloomery was charged with charcoal and iron ore and then lit. Air was blown in through a tuyere to heat the bloomery to a temperature somewhat below the melting point of iron. In the course of the smelt, slag would melt and run out, and carbon monoxide from the charcoal would reduce the ore to iron, which formed a spongy mass. The iron remained in this solid state. If the bloomery was allowed to become hot enough to melt the iron, carbon would dissolve into it and form pig or cast iron, but that was not the intention.
Wrought iron was originally produced by a variety of smelting processes, all described today as bloomeries. Different forms of bloomery were used at different places and times. The bloomery was charged with charcoal and iron ore and then lit. Air was blown in through a tuyere to heat the bloomery to a temperature somewhat below the melting point of iron. In the course of the smelt, slag would melt and run out, and carbon monoxide from the charcoal would reduce the ore to iron, which formed a spongy mass. The iron remained in this solid state. If the bloomery was allowed to become hot enough to melt the iron, carbon would dissolve into it and form pig or cast iron, but that was not the intention.
After smelting was complete, the bloom was removed, and the process could then be started again. It was thus a batch process, rather than a continuous one. The spongy mass contained iron and also silicate (slag) from the ore; this was iron bloom from which the technique got its name. The bloom had to be forged mechanically to consolidate it and shape it into a bar, expelling slag in the process.
During the Middle Ages, water-power was applied to the process, probably initially for powering bellows, and only later to hammers for forging the blooms. However, while it is certain that water-power was used, the details of this remain uncertain. This was the culmination of the direct process of ironmaking. It survived in Spain and southern France as Catalan Forges to the mid 19th century, in Austria as the stückofen to 1775, and near Garstang in England until about 1770; it was still in use with hot blast in New York State in the 1880s.
Osmond Process
Osmond iron consisted of balls of wrought iron, produced by melting pig iron and catching the droplets on a staff, which was spun in front of a blast of air so as to expose as much of it as possible to the air and oxidize its carbon content. The resultant ball was often forged into bar iron in a hammer mill.
Finery Forge in the 15th Century
The blast furnace spread into what is now Belgium and was improved. From there, it spread via the Pays de Bray on the boundary of Normandy and then to the Weald in England. With it, the finery forge spread. These remelted the pig iron and (in effect) burnt out the carbon, producing a bloom which was then forged into a bar iron. If rod iron was required, a slitting mill was used.
The finery process existed in two slightly different forms. In Great Britain, France, and parts of Sweden, only the Walloon process was used. This employed two different hearths, a finery hearth for fining the iron and a chafery hearth for reheating it in the course of drawing the bloom out into a bar. The finery always burnt charcoal, but the chafery could be fired with mineral coal, since its impurities would not harm the iron when it was in the solid state. On the other hand, the German process, used in Germany, Russia, and most of Sweden used a single hearth for all stages.
The introduction of coke for use in the blast furnace by Abraham Darby
In 1709 (or perhaps others a little earlier) initially had little effect on wrought iron production. Only in the 1750s was coke pig iron used on any significant scale as the feedstock of finery forges. However, charcoal continued to be the fuel for the finery.
Potting and stamping from the late 1750s - Ironmasters began to develop processes for making bar iron without charcoal. There were a number of patented processes for this, which are referred to today as potting and stamping. The earliest were developed by John Wood of Wednesbury and his brother Charles Wood of Low Mill at Egremont, patented in 1763. Another was developed for the Coalbrookdale Company by the Cranage brothers. Another important one was that of John Wright and Joseph Jesson of West Bromwich.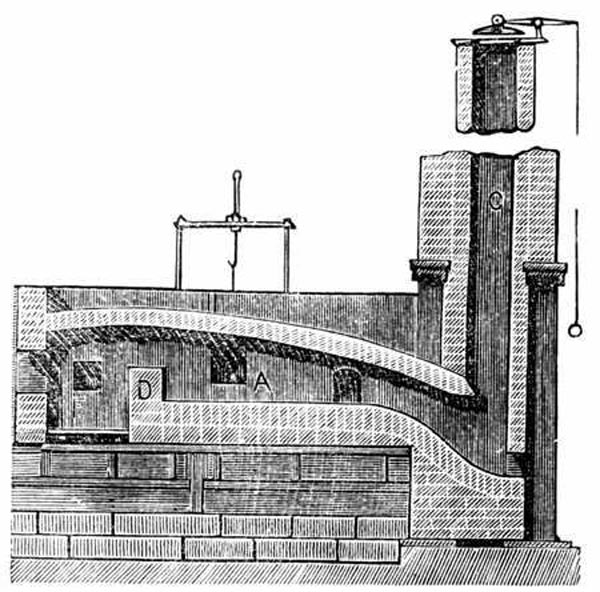
Schematic Drawing of a Puddling Furnace Puddling (metallurgy)
A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of these was puddling, using a puddling furnace (a variety of the reverberatory furnace). This was invented by Henry Cort in 1784. It was later improved by others including Joseph Hall. In this type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by impurities in it. The flame from the fire is reverberated or sent back down onto the metal on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw material first had to be refined into refined iron or finers metal. This would be done in a refinery where raw coal is used to remove silicon and convert carbon from a graphitic form to a combined form. This metal was placed into the hearth of the puddling furnace where it was melted. The hearth was lined with oxidizing agents such as haematite and iron oxide. This mixture is subjected to a strong current of air and stirred with long bars, called puddling bars or rabbles, through working doors. The air, stirring, and "boiling" action of the metal help the oxidizing agents to oxidize the impurities and carbon out of the pig iron to their maximum capability. As the impurities oxidize, the retaining material solidifies into spongy wrought iron balls, called puddle balls.
Shingling (metallurgy)
There is still some slag left in the puddle balls so while they are still hot they must be shingled to remove the remaining slag and cinder. It may be achieved by forging the balls under a power hammer or by squeezing the bloom in a machine. The material obtained at the end of shingling is known as bloom and it is still red-hot. The blooms are not useful in this form so they must be rolled into a final product.
Sometimes European ironworks would skip this step completely and roll the puddle balls. The only drawback to this is that the edges of the rough bars are not as well compressed. When the rough bar is reheated, the edges may separate and be lost into the furnace.
Rolling mill
The bloom is passed through grooved rollers and flat bars were produced. These bars of wrought iron were of poor quality, called muck bars or puddle bars. To improve the quality of wrought iron, these bars were cut up, piled and tied together by wires, a process known as faggoting or piling. They were then reheated and rolled again in merchant rolls. This process may be repeated several times to get wrought iron of desired quality. Wrought iron that has been rolled multiple times is called merchant bar or merchant iron.
Lancashire Process
 The advantage of puddling was that it used coal, not charcoal as fuel. However this was little advantage in Sweden, which lacks coal. Gustaf Ekman observed charcoal fineries at Ulverstone, which were quite different from any in Sweden. After his return to Sweden in the 1830s, he experimented and developed a process similar to puddling but using firewood and charcoal, which was widely adopted in the Bergslagen in the following decades.
The advantage of puddling was that it used coal, not charcoal as fuel. However this was little advantage in Sweden, which lacks coal. Gustaf Ekman observed charcoal fineries at Ulverstone, which were quite different from any in Sweden. After his return to Sweden in the 1830s, he experimented and developed a process similar to puddling but using firewood and charcoal, which was widely adopted in the Bergslagen in the following decades.
The Aston Process
In 1925, James Aston of the United States developed a process for manufacturing wrought iron quickly and economically. It involves taking molten steel from a Bessemer converter and pouring it into cooler liquid slag. The temperature of the steel is about 1500 °C and the liquid slag is maintained at approximately 1200 °C. The molten steel contains a large amount of dissolved gases so when the liquid steel hits the cooler surfaces of the liquid slag the gases are liberated. The molten steel then freezes to yield a spongy mass having a temperature of about 1370 °C. This spongy mass must then be finished by being shingled and rolled as described under puddling (above). Three to four tons can be converted per batch with this method.
Wrought iron is no longer commercially produced. The last wrought iron facility shut down in 1969. In the 1960s the price of steel production was dropping due to recycling and even using the Aston process wrought iron production was a labor intensive process. It has been estimated that the production of wrought iron costs approximately twice as much as the production of low carbon steel.
The microstructure of wrought iron shows dark slag inclusions in ferrite (iron). The slag inclusions in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch. A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) in order to harden them. An advantage of its low carbon content is its excellent weldability. Furthermore, sheet wrought iron cannot bend as much as steel sheet metal (when cold worked).
Wrought Iron Can Be Cast
However, there is no engineering advantage as compared to cast iron; cast iron is much easier to produce and thus cheaper, so it is exclusively chosen over wrought iron.
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance compared to other iron alloys. There are many mechanisms behind this corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion. They also found that in puddled and forged and piled the working over of the iron spread out copper, nickel and tin impurities, which produce electrochemical conditions that slow down corrosion.
The slag inclusions have been shown to disperse corrosion in to an even film to resist pitting. Another study has shown that slag inclusions are pathways to corrosion. Other studies show that sulfur impurities in the wrought iron decrease corrosion resistance, but phosphorus increase corrosion resistance. Environments with a high concentration of chlorine ions also decreases wrought iron's corrosion resistance.
Wrought iron has a rough surface so it can hold platings and coatings better. For instance, a galvanic zinc finish is approximately 25"40% thicker than the same finish on steel. In Table 1, the chemical composition of wrought iron is compared to that of pig iron and carbon steel. Although it appears that wrought iron and plain carbon steel have similar chemical compositions, this is deceiving. Most of the manganese, sulfur, phosphorus, and silicon are incorporated into the slag fibers present in the wrought iron, so wrought iron really is purer than plain carbon steel.
Understanding True Quality Iron Working Makes All the Difference
When you purchase investment quality furnishings, doors, gates, cabinets, lighting, and hardware, they will appreciate and either keep up with inflation or exceed most other investments. For this reason, becoming wealthy has very little to do with a higher education or having a lot of extra money, it is making truly wise decisions and a state of mind that allows for you to pay once for a good value and continue to grow wealth while enjoying living with your investment. Not to mention this type of investing has many other benefits, such as owning priceless family heirlooms that have meaning and the ability to pass on your legacy to future generations.
In conclusion, if you are in the market for the best quality, hand made in America, solid, hand forged ornamental iron cabinet knobs, entrance door handles, entrance gates, iron fencing, iron furniture, hand forged lighting or furnishings for your residential or commercial project, then the facts speak for themselves -- ArtFactory.com is the right choice - call 1-800-292-0008.
We Offer Our Facility to Advance Go Green Technology and The Arts
We offer use of our facility and the sharing of the experience of our master craftsmen in return for these contributions when available, thus allowing for hands-on experience training in the lost arts. We also allow use of our facility for project development in related fields under any accredited school program. Products produced in these programs are sold and proceeds are used to fund the advancement of these programs with no weight to profit.
We also offer the use of our facility for the advancement of environmental energy saving designs in connection with government funded Go Green development. These energy saving designs must be associated with our natural material, building projects such as doors/windows etc. without effecting the artistic value. This is a not-for-profit program provided by ArtFactory.com in the hopes of advancing new technology in conjunction with arts and crafts as it relates to our contribution to the world of fine art craftsmanship.
We strive to acquire all correct historical knowledge available related to the subject of blacksmith, ironworks, and metallurgy, so please contact us with any corrections or additions to this educational page.
Source: https://wikipedia.org
Scientific Properties of Metals
Table 1: Chemical composition comparison of pig iron, plain carbon steel, and wrought iron
Table 2: Properties of wrought iron
7.5-7.8
Amongst its other properties, wrought iron becomes soft at red heat, and can be easily forged and forge welded. It can be used to form temporary magnets, but cannot be magnetized permanently, and is ductile, malleable and tough.